
Ginger use dates back to as early as 400 BC, as both a culinary and medicinal spice in Asian cultures. Its primary therapeutic use involved remedying various upper gastrointestinal system issues, from dyspepsia and gastritis to indigestion and nausea. Ginger’s volatile oils, contributing at only 2-3% of the rhizome’s total composition, have been identified as the active involved in providing this relief. Though gingeroids (gingerols and shogaols) naturally make up about 50% of ginger’s volatile oil content, their overall low assay, lack of stability and lipophilic nature have resulted in limited clinical evidence, or evidence only with very high dosing (e.g. 2-4 g/day).
Ginfort® delivers more than 12X the gingeroid and 6-gingerol content of traditional ginger rhizome preparations, without any solvents and with a proven stability, above specification over 2+ years.

The patented Ginfort® advantage is availed from the application of Aqueosome® technology. This platform enhances the solubility of lipophilic gingeroids from the ginger oleoresin through the patented application of a blend of safe, food-grade inactives. This innovative step frees Ginfort® from consumer-adverse bioenhancers like polysorbate and PVP, as well as absolving it from the unrestrained use of maltodextrin, as is common in spray-dried formats.

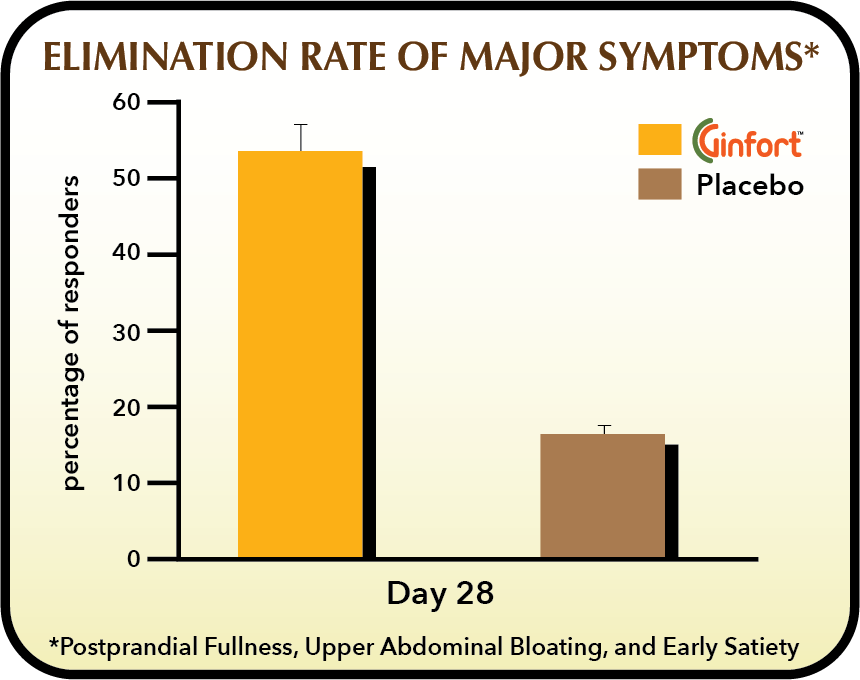
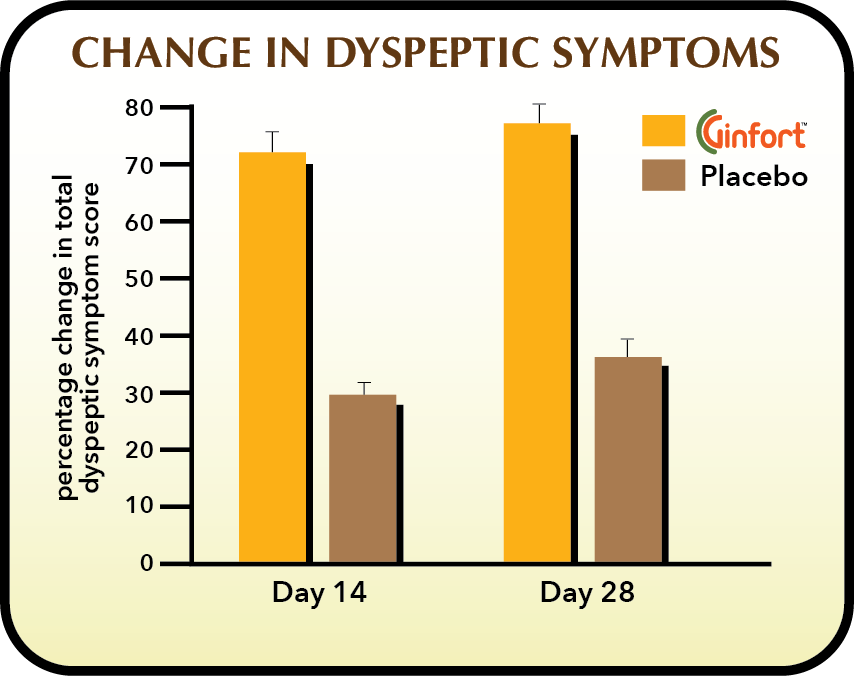
Gingeroids have carminative action – reducing gas, flatulence and bloating. They are also proposed to act as a prokinetic, promoting synchronized peristalsis, over dysfunctional stomach and small intestine motility – common abnormalities at the root of functional dyspepsia.
Ginfort®, taken in 200 mg capsules 2-times/day, was trialed in a 28-day double-blind, randomized controlled trial in subjects with Functional Dyspepsia. Interim benefits were also assessed at day 14 for a primary outcome of overall treatment efficiency as measured on a 7 point Likert Scale and secondarily, the elimination rate for three primary functional dyspepsia symptoms. Safety data was also collected and it was concluded that Ginfort® intake was without consequence on any safety parameter.
Ginfort® is a registered trademark of Olene Life Sciences Pvt. Ltd., exclusively licensed to DolCas Biotech, LLC.
Aqueosome® is a registered trademark of Olene Life Sciences Pvt. Ltd.

Applications
Your Subtitle Goes Here
- Powder for encapsulation
- Lozenges, candies
Benefits
Your Subtitle Goes Here
- Supports gut health
- Promotes healthy digestion
- Improves upper-abdominal bloating
- Reduces digestive upset
- Relieves excessive belching
- Helps occasional heartburn symptoms
- Supports joint health*
- Improves nausea and vomiting*
- Helps pregnancy sickness*
* Generally supported by the literature
Composition
Your Subtitle Goes Here
Safety
Your Subtitle Goes Here
Clinical Research
Your Subtitle Goes Here
Want to Learn More?
Please leave us your contact information below for a free brochure.
Our Products
TruOliv® is a genuine olive fruit and leaf-sourced, waste-water and solvent FREE, USDA and EU organic, polyphenol-rich powder extract with clinically proven benefits.
MoriKol® is a clinically-proven, fast-acting, bioavailable marine collagen. A low dose supplys a minimum of 15% tripeptides for the convenient delivery of beauty from within bioactives.
MMP50® is a New-Zealand sourced green lipped mussel extract, uniquely crafted, rich in Omega-3's and standardized for bioactivity in joint health using a MMP-inhibition assay.
Bergacyn®FF is a patented, clinically-proven Bergamot and Cynara leaf extract, supporting cardiovascular and metabolic health in populations having accumulated excess liver fat.
Truly ‘next GENeration’ Curcugen® is a clinically-studied, 39X-bioavailable, turmeric oleoresin-based curcumin extract with a food & beverage friendly dispersion and taste profile.
Fortiquin® is a patent-pending standardized formulation of herbs and nutrients that are clinically proven to support male sexual stamina and overall well-being.
Ginfort® is a patented, highly stable, concentrated gingeroids (26%) active. The solvent-free extract has clinically-proven benefits in relieving symptoms of functional dyspepsia.










