
Curcuminoids, turmeric essential oils and resins are naturally housed within the turmeric oleoresin. They are exclusively represented in Curcugen® and complemented by a diverse spectrum of other turmeric native compounds. Curcugen’s full-spectrum profile benefits from the strategic and patent-protected application of safe, food-grade solvents and precipitants.
Curcugen® is the only oleoresin-sourced turmeric active on the market to generate its absorption benefit from the dispersion power found in a previously ignored turmeric native – ‘polar’ resins.
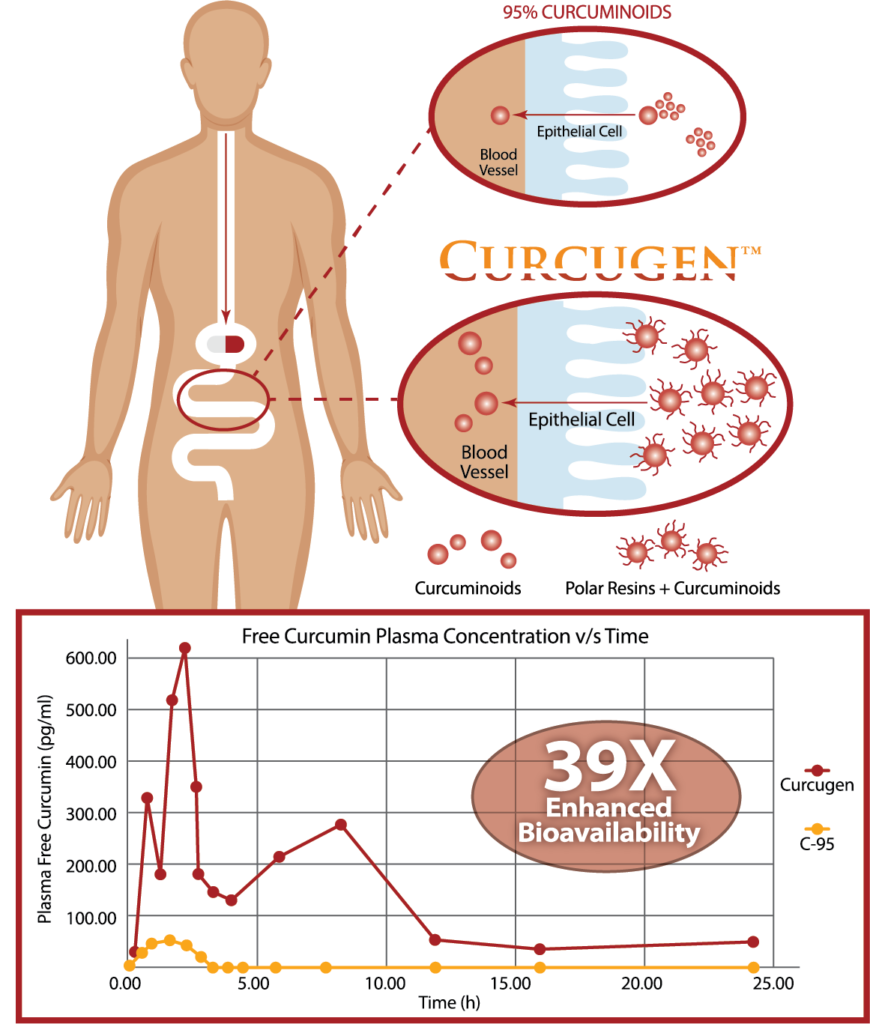
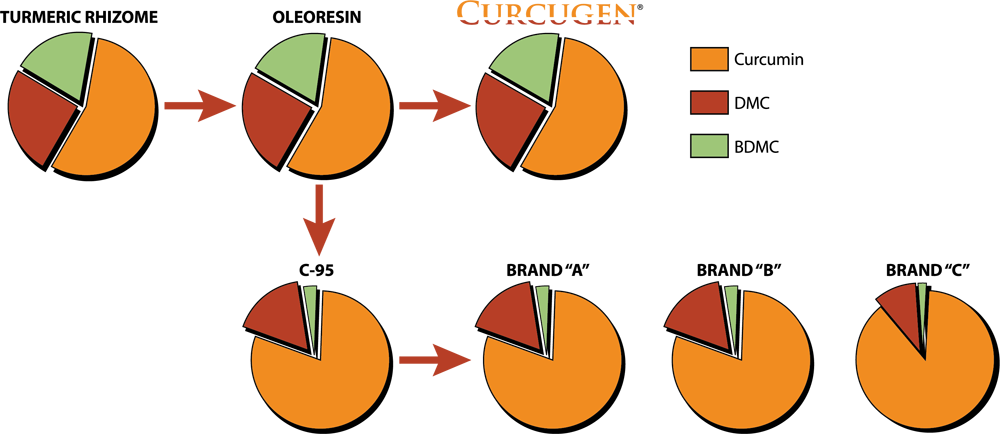
Curcugen® uniquely delivers the same ratio of curcuminoid analogues – curcumin, demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC) – as found in the turmeric rhizome in its food state, or as its primary derivative, the oleoresin. While Curcugen® is concentrated to a 50% standard of curcuminoids, it is not hyper-purified, as is common with other curcuminoid formulations. Therein, Curcugen® retains a closely matched ratio of these cousin-molecules to what is natural, and found most familiarly in food.
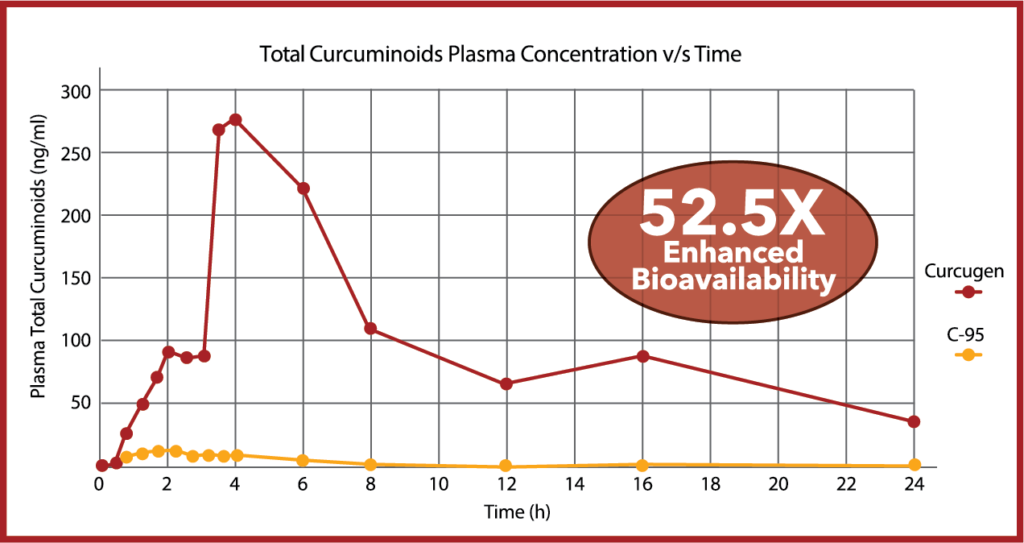
In an 18-subject, cross-over pharmacokinetics study over 24-hours, Curcugen®, at 2000 mg of curcuminoids equivalents enhanced FREE-curcumin bioavailability by 39X that of placebo, and proved 52.5-times more bioavailable for Total Curcuminoids than standardized curcumin 95% (C-95).
Curcugen® is one of the first curcuminoids formulas to prove an increase in the bioavailability of endogenously-produced tetrahydrocurcumin (THC). THC is thought to be a more potent antioxidant than curcumin, although curcuminoid analogues appear to still offer more potent inflammatory signal modulation than THC. Curcugen® enhanced total THC bioavailability by 31X over C-95.
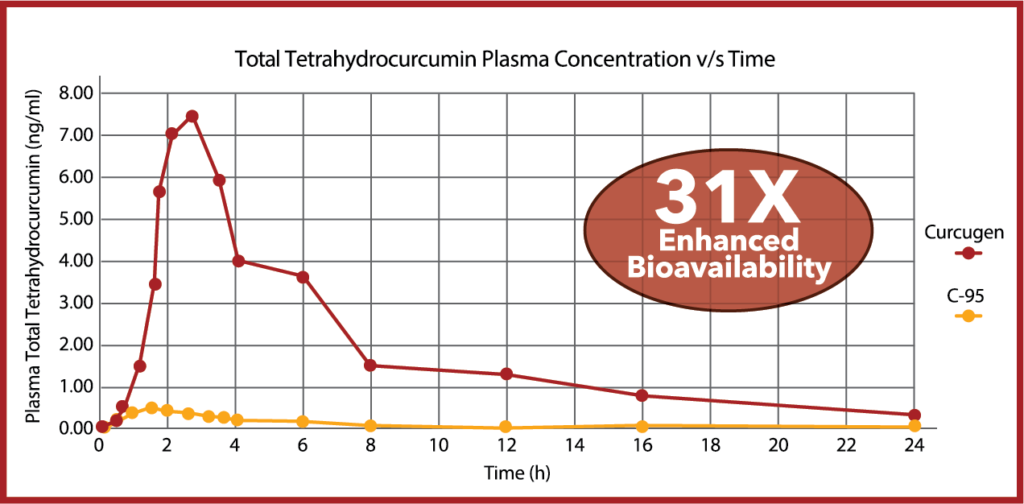
Curcugen’s efficacy is backed by several, published clinical studies in the dosage range of 500 – 1000 mg/day. The Curcugen study portfolio will continue to grow with additional clinical studies underway or in active peer review. Please contact our team for periodic updates.

Curcugen® is a suitable for a variety of formulations – traditional and novel food and beverage formats alike. Curcugen® is palate-friendly – requiring minimal flavoring and no masking, free flowing, highly dispersible and very stable. Many formulation concepts for Curcugen® have been explored, some of which include:

Applications
Your Subtitle Goes Here
- Encapsulation
- Tableting
- Ready to drink powder
- Ready to mix powder
- Oral dispersible powder
- Fast melts
- Soft chews
- Chocolate
- Hard candy/lozenges
Benefits
Your Subtitle Goes Here
- 39-times enhanced bioavailability (Free Curcumin)
- Supports overall well-being
- Antioxidant
- Reduces exercise-induced pain and discomfort
- Supports immunity
Composition
Your Subtitle Goes Here
- 98.5% turmeric-oleoresin extract
- 1.5% flow agent
Safety
Your Subtitle Goes Here
- Curcugen® has an LD50 > 5,000 mg/kg in an animal model, establishing it as a safe ingredient in an acute, single-dose model.
- A 90-day, chronic, repeat dose OECD-guided toxicity study in an animal model has also proven Curcugen® to be safe > 2000 mg/kg/day.
- Curcugen® has also proven not to produce genotoxicity or mutagenicity, as per OECD-testing procedures.
Clinical Research
Your Subtitle Goes Here
An extensive clinical study program for Curcugen® evaluating its efficacy at doses between 500-1000 mg/day has been initiated at both domestic and international universities and contract research organizations. The below list references those studies that have already been published. Contact our sales team to learn more about those that are ongoing.
- Swartz N, et al. A double-blinded, placebo-controlled trial evaluating the antiinflammatory effects of Curcugen in an acute exercise model. Journal of the International Society of Sports Nutrition 2020, 17(Suppl 2):A22.
Want to Learn More?
Please leave us your contact information below for a free brochure.
Our Products
TruOliv® is a genuine olive fruit and leaf-sourced, waste-water and solvent FREE, USDA and EU organic, polyphenol-rich powder extract with clinically proven benefits.
MoriKol® is a clinically-proven, fast-acting, bioavailable marine collagen. A low dose supplys a minimum of 15% tripeptides for the convenient delivery of beauty from within bioactives.
MMP50® is a New-Zealand sourced green lipped mussel extract, uniquely crafted, rich in Omega-3's and standardized for bioactivity in joint health using a MMP-inhibition assay.
Bergacyn®FF is a patented, clinically-proven Bergamot and Cynara leaf extract, supporting cardiovascular and metabolic health in populations having accumulated excess liver fat.
Truly ‘next GENeration’ Curcugen® is a clinically-studied, 39X-bioavailable, turmeric oleoresin-based curcumin extract with a food & beverage friendly dispersion and taste profile.
Fortiquin® is a patent-pending standardized formulation of herbs and nutrients that are clinically proven to support male sexual stamina and overall well-being.
Ginfort® is a patented, highly stable, concentrated gingeroids (26%) active. The solvent-free extract has clinically-proven benefits in relieving symptoms of functional dyspepsia.










