Bergacyn®FF is a patented extract blend of bergamot citrus and Cynara cardunculus, clinically proven to support normal liver fat levels and promote healthy weight loss in overweight individuals. Native to Italy’s southern coasts, Bergacyn®FF actives grow in a unique microclimate fostered by the abrupt contrast of the Appinnine Mountains and the Mediterranean Sea.

Excess liver fat accumulation and its consequences are a growing concern. Majorly driven by processed food intake, excess calories and sedentary lifestyle, fatty liver is estimated to affect over 25% of the population with aggressive growth predictions for the future, as Metabolic Syndrome and obesity reach all-time highs. Bergacyn®FF delivers an exclusive profile of polyphenols and sesquiterpenes that facilitate the ingredient’s action in promoting healthier liver fat metabolism and modulating the immune triggers that instigate downstream liver damage.
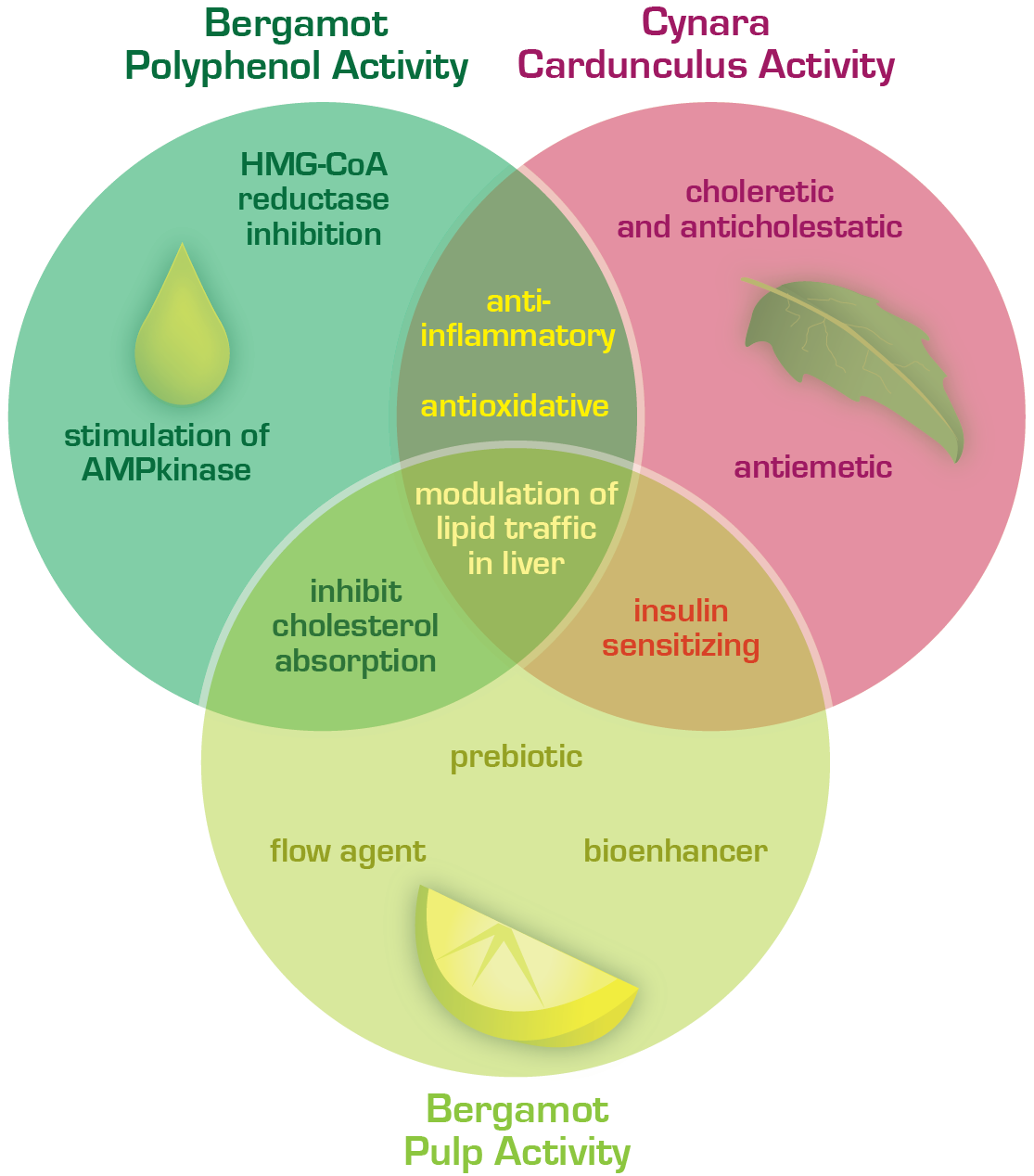
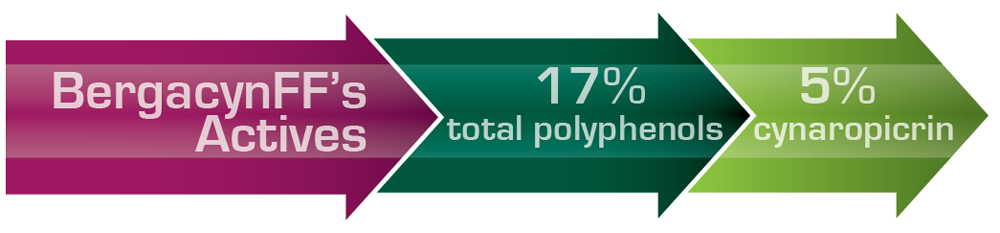
BergacynFF’s exclusive use of bergamot albedo (pulp) fibers and sesquiterpenes from the Cynara leave combine novelly to round out the rich polyphenol profile of 17% polyphenols, contributed to by all the formulation’s components. The pulp fibers act as synergistic bioenhancers (delivering 2.5-times more) polyphenols from both bergamot and cynara, as would be availed otherwise. Lastly, the bergamot pulp fibers establish the formulation as 100% excipient-FREE, appealing to clean-label formulations and uncomplicated production runs.

BergacynFF’s enhanced profile gives it a competitive edge in dosing over other common liver fat support actives and ingredients that support healthy metabolism. Many common hepatoprotective nutrients and botanicals oblige doses well over 1,000 mg per day, whereas Bergacyn®FF has been trialed and proven effective at only 600 mg per day.
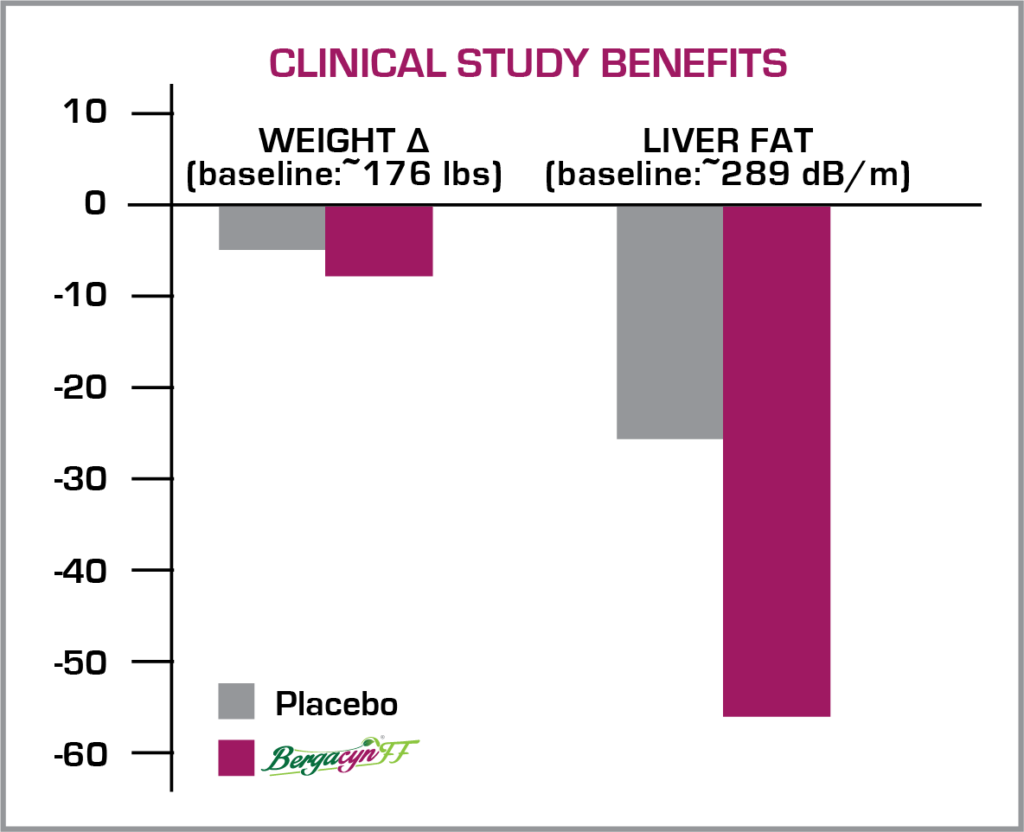
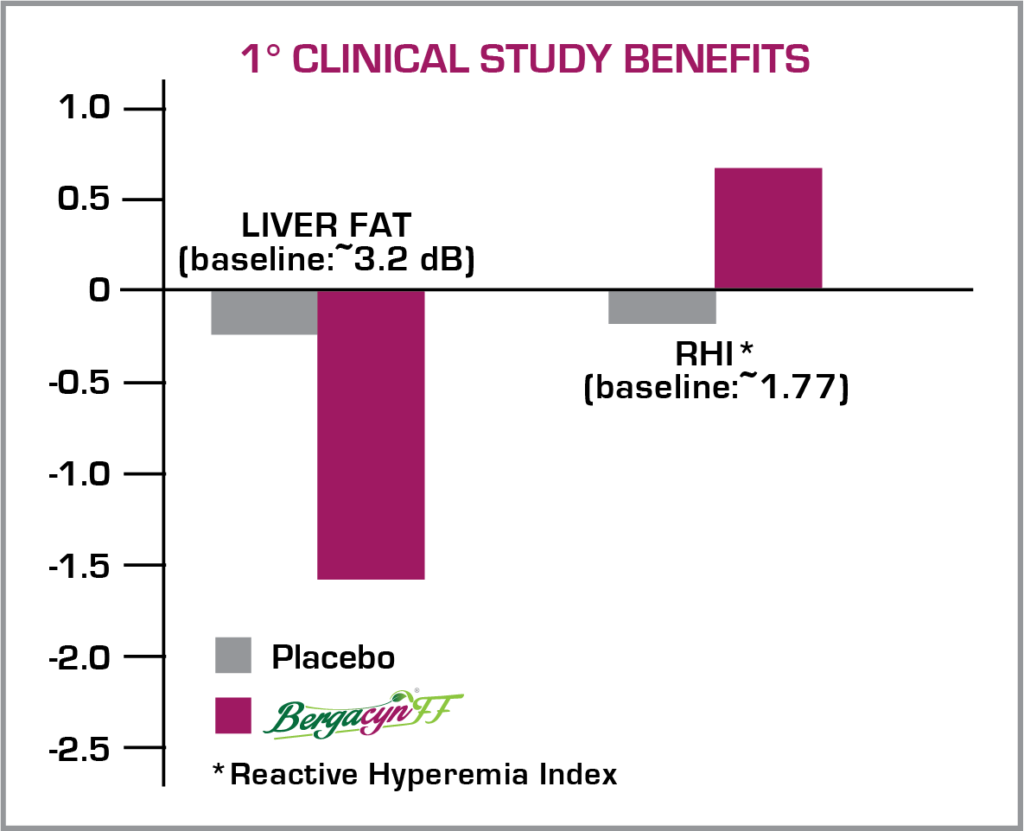
Bergacyn®FF was trialed in 2 published, clinical studies. Study #1 evaluated Bergacyn®FF at 600 mg/day over 12 weeks in subjects with Metabolic Syndrome and increased baseline liver fat levels. Bergacyn®FF more effectively attenuated liver fat levels and body weight than placebo, even with imposed baseline BMI-assigned caloric restrictions in both groups.
The 2nd Clinical Study on Bergacyn®FF trialed the formulation in a Diabetic population with excessive liver fat accumulation and borderline endothelial dysfunction. This study also evaluated the synergism potential of the Bergacyn®FF formula over standalone bergamot or Cynara extracts of the same dose. Many other dysmetabolism secondary endpoints were also evaluated in this trial.
Bergacyn®FF is a registered trademark of H&AD srl, exclusively licensed to DolCas Biotech, LLC.
Applications
Your Subtitle Goes Here
- Free-flowing material
- Formulation for softgels
Benefits
Your Subtitle Goes Here
- Supports healthy liver fat levels
- Supports healthy lipid levels
- Supports weight loss/weight management
- Supports vascular function
- Promotes healthier metabolism
Composition
Your Subtitle Goes Here
Safety
Your Subtitle Goes Here
Bergacyn®FF is a safe and well-tolerated ingredient, evaluated for safety in animal models and within a human clinical trial. Bergacyn®FF has an LD50 > 5,000 mg/kg and showed no histopathological toxicity, over 90 days in an OECD-guided chronic rat model. Further, Bergacyn®FF was subject to mutagenicity and cytotoxicity study and showed no toxic effects. In the human clinical trial, evaluating 102 subjects for BergacynFF’s effects on lipid accumulation in the liver, BergacynFF’s intake in susceptible populations showed the lowest risk of worsening or not improving of liver fat accumulation progression, as compared to placebo. Further, the prevalence of adverse events were not any greater than those resulting from the placebo, all of which were of low incidence and of grade 1 quality.
Clinical Research
Your Subtitle Goes Here
Liver, Cardio-metabolic, and Weight Management Support
Bergacyn®FF has demonstrated highly efficacious results at only 600 mg/day. Two studies have been published on Bergacyn®FF effect in dysmetabolic subjects as well as in clinically diagnosed, Type 2 Diabetes, over 12 and 14 weeks respectively. Bergacyn®FF continues to be studied in ongoing clinical investigations. The below studies have already been published.
- Musolino V, et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. May 2020; 10(2): 268-274
FAQ
Your Subtitle Goes Here
Coming soon…
Want to Learn More?
Please leave us your contact information below for a free brochure.
Our Products
TruOliv™ is a genuine olive fruit and leaf-sourced, waste-water and solvent FREE, USDA and EU organic, polyphenol-rich powder extract with clinically proven benefits.
MoriKol® is a clinically-proven, fast-acting, bioavailable marine collagen. A low dose supplys a minimum of 15% tripeptides for the convenient delivery of beauty from within bioactives.
MMP50™ is a New-Zealand sourced green lipped mussel extract, uniquely crafted, rich in Omega-3's and standardized for bioactivity in joint health using a MMP-inhibition assay.
Bergacyn®FF is a patented, clinically-proven Bergamot and Cynara leaf extract, supporting cardiovascular and metabolic health in populations having accumulated excess liver fat.
Truly ‘next GENeration’ Curcugen® is a clinically-studied, 39X-bioavailable, turmeric oleoresin-based curcumin extract with a food & beverage friendly dispersion and taste profile.
Fortiquin™ is a patent-pending standardized formulation of herbs and nutrients that are clinically proven to support male sexual stamina and overall well-being.
Ginfort® is a patented, highly stable, concentrated gingeroids (26%) active. The solvent-free extract has clinically-proven benefits in relieving symptoms of functional dyspepsia.











